38
|
CERECDOCTORS.COM
|
QUARTER 2
|
2015
| | |
B O LT C H I
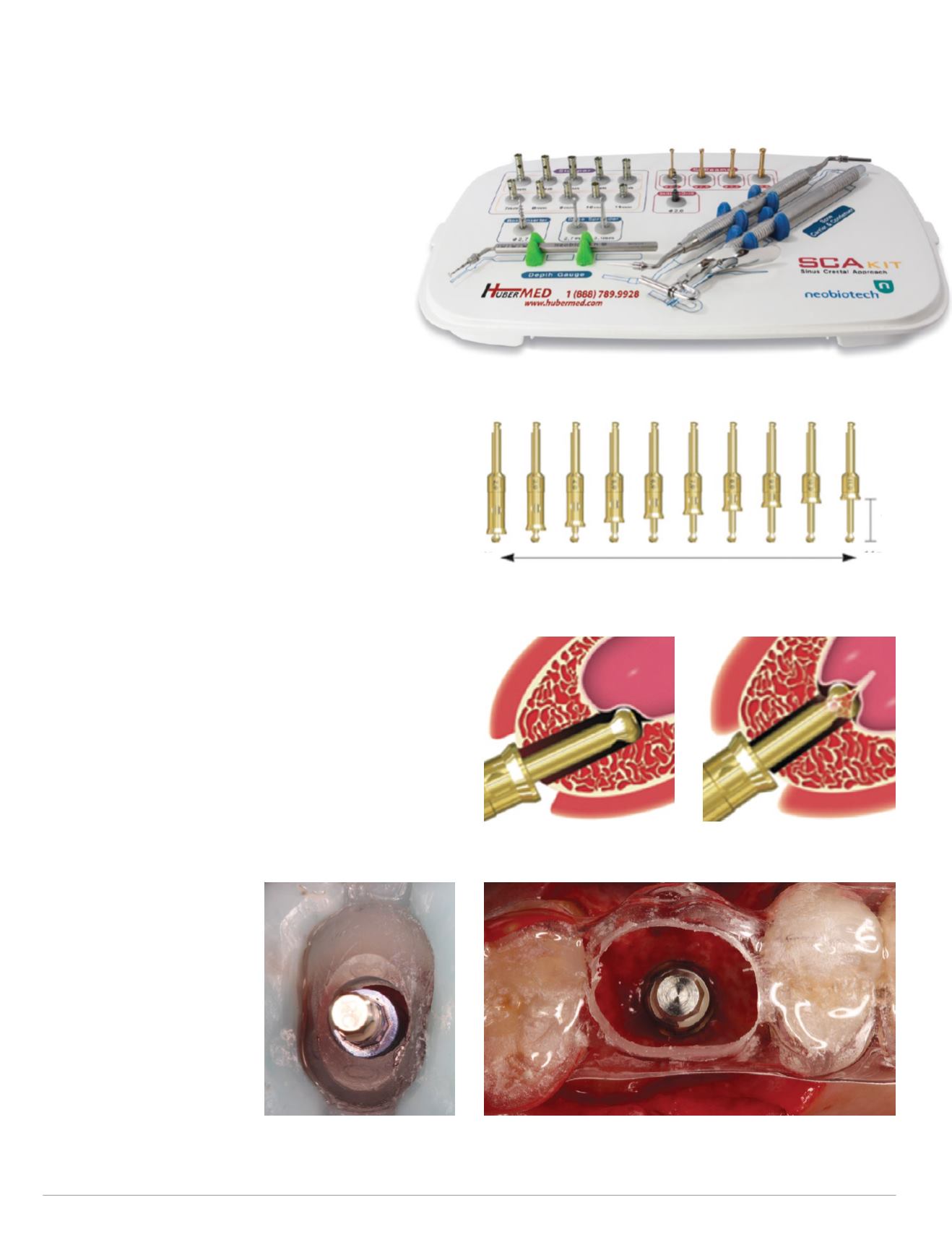
TheCERECGuidewas then removed and theNeobio-
tech SCA kit (Fig. 11) was utilized to perform the neces-
sary transcrestal sinus floor elevation. The special SCA
S-Reamer bur with the corresponding stoppers was
used to gain access to themaxillary sinuswithout perfor-
ating the Schneiderian sinus membrane (Figs. 12-13).
The PRF plugs were cut into smaller pieces, and
several of the pieces were mixed with a slow-resorbing
anorganic bovine bone graft material. The Straumann
transcrestal sinus floor elevation osteotomes were then utilized to
introduce the bone graft into the sinus in small increments alter-
nating between the Bio-Oss bone graft/PRF mixture and the PRF
plugs alone. Each increment of bone graft/PRF mixture and PRF
plug corresponds to approximately 1 mm of sinus floor elevation,
and care must be taken to perform this procedural step slowly
to avoid the perforation and tearing of the sinus Schneiderian
membrane.
The SCA bone spreader with the corresponding stopper was
then used to spread the bone graft material in the sinus laterally
and, after obtaining approximately 5 mm of sinus floor elevation,
the CEREC Guide was re-inserted and the final 4.2 mm guided
implant osteotomy preparation was performed. This was done
with a slight modification of the guided drilling protocol to allow
this final guided drill to penetrate the sinus by 1 mm to 2 mm.
There is no risk of perforating the sinus membrane at this time,
since the inserted bone graft material is protecting the elevated sinus
membrane. A final increment of Bio-Oss bone graft/PRF mixture
and PRF plug was placed in the osteotomy and a Straumann 4.8
mm X 10 mm SLActive Bone Level implant was placed through the
CEREC Guide. This was done in a semi-guided fashion in the restor-
atively correct and pre-planned position and acting as the final bone
graft carrier (Figs. 14-15). The
implant was placed 0.5 mm to
1 mm subcrestally, achieved
excellent primary stability, and
was placed within the confines
of the osseous alveolar housing
without any associated buccal
or palatal dehiscence defects
(Fig. 16).A2-mmconicalhealing
capwas placed onto the implant
and a buccal and palatal contour
bone graft augmentation was
performed with the Bio-Oss/
PRF bone graft mixture, which
2mm Stopper
11mm Stopper
11mm
Fig. 12: SCA kit 2-11mm drill stoppers
Fig. 13: SCA kit S-Reamer special drill with stopper
Fig. 14: Implant site preparation
with CEREC Guide
Fig. 11: Neobiotech SCA kit
Fig. 15: Restoratively driven implant placement site #3


